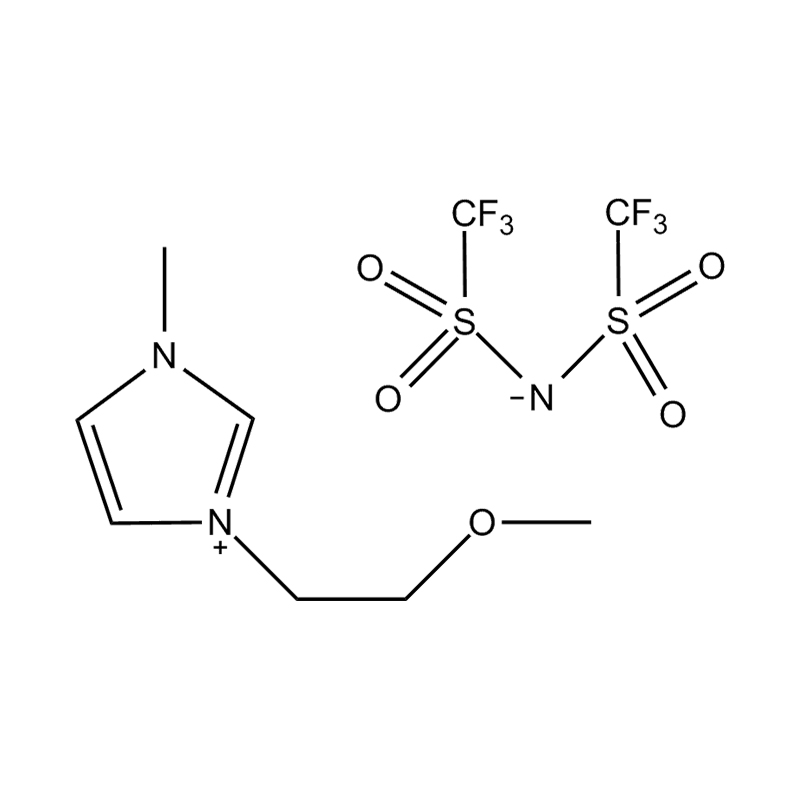
The chemical structure of 1-methoxyethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide salt consists of an imidazole ring, which is a five-membered ring consisting of a nitrogen atom and a carbon atom, to which a methoxyethyl and a methyl group are attached. The imidazole ring also has a bis(trifluoromethanesulfonyl)imide group attached to one side, which is negatively charged.
Its physical properties include a colourless to pale yellow liquid with a density of about 1.53 g/cm³, a melting point above -15°C, a boiling point of about 543.6°C, a refractive index between 1.4220 and 1.4260, and a flash point of more than 200°C. It has a vapour pressure of 0.004-0.007 Pa at 140.9-150.9°C and is insoluble in water. soluble in water.
In terms of application, this compound can be used in the field of electrochemistry, for example as an electrolyte in batteries or capacitors. It is highly conductive, chemically stable and thermally stable, which makes it potentially useful in areas such as high-performance batteries and supercapacitors. Proper safety measures need to be taken when working with this compound as it can be irritating to the skin and eyes and toxic to aquatic organisms. So, always remember to wear protective gloves and goggles if you are using it in the lab.


 English
English Deutsch
Deutsch Español
Español 中文简体
中文简体