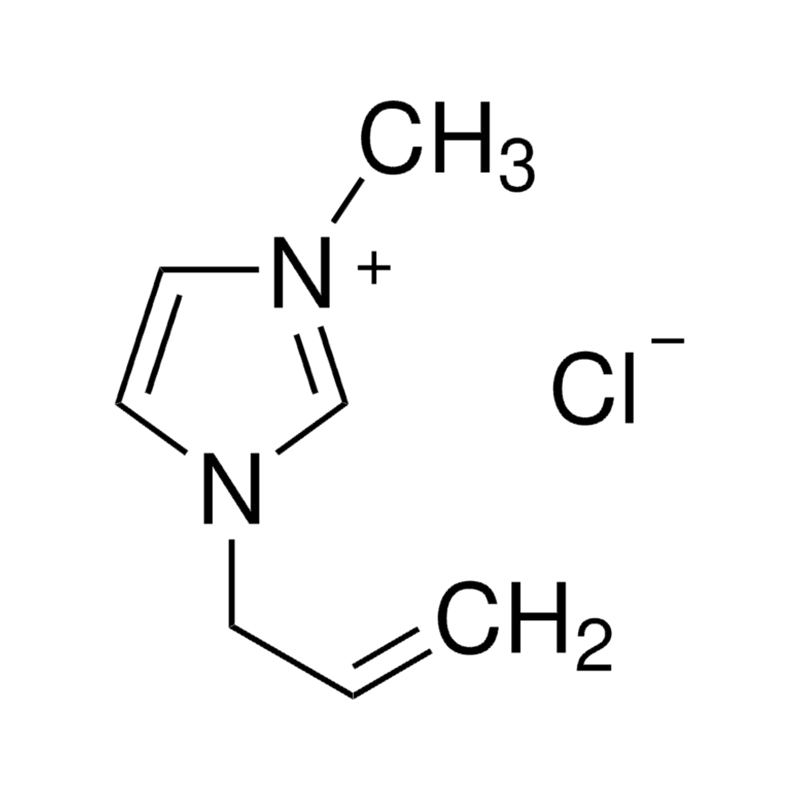
The cationic portion of the 1-allyl-3-methylimidazole chloride salt consists of 1-allyl-3-methylimidazolyl groups with allyl substituents that add flexibility to the molecular structure. The allyl group is able to provide a certain degree of nucleophilicity and reactivity, and has a unique role in certain organic reactions, such as in olefin-related polymerisation or copolymerisation reactions. Imidazolium-based ionic liquids have excellent hydrogen bond donor properties due to their cationic structure and can form stable interactions with other molecules through hydrogen bonding networks. It shows good solvent properties when dissolving cellulose or other polar polymers, especially during biomass treatment and cellulose dissolution. It has a melting point of 52°C, appears as a solid at slightly above room temperature, has a low conductivity, a viscosity of 150 cP, is less fluid in the liquid state, has a thermal decomposition temperature of 180°C, and needs to be stored in sealed containers in a cool, dry place. The storage place must be far away from oxidising agents and avoid moisture and water sources. -20ºC Storage.
What Are Solid Electrolytes and Why Do They Matter? Solid electrolytes are the defining component of solid-state batteries, replacing the liquid or gel electrolytes found in conventional lithium-ion cells. In a tradition...
READ MORE

 English
English Deutsch
Deutsch Español
Español 中文简体
中文简体