In the ever-evolving landscape of advanced materials, ionic liquids (ILs) have emerged as a revolutionary class of substances that defy conventional categorizations of liquids, salts, and solvents. But what exactly makes ionic liquids so unique—and why are they increasingly regarded as a cornerstone in the development of sustainable technologies, green chemistry, and next-generation electrochemical systems?
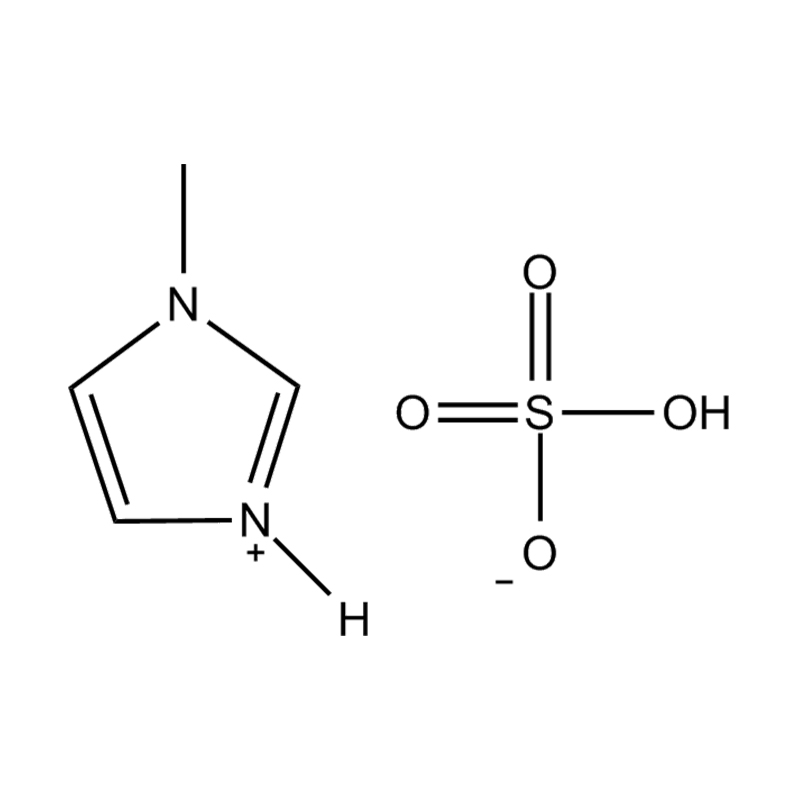
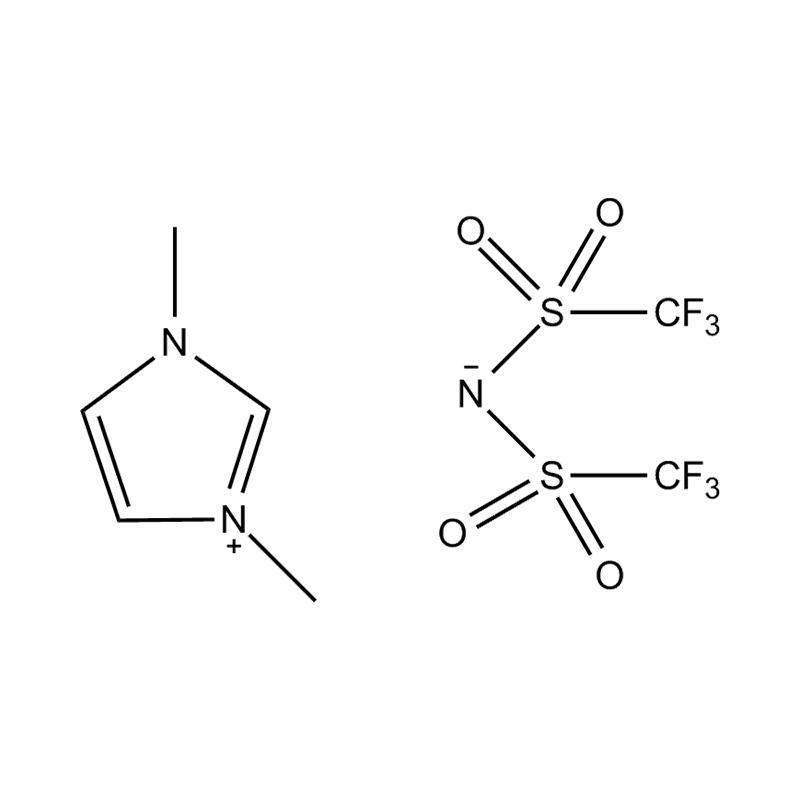
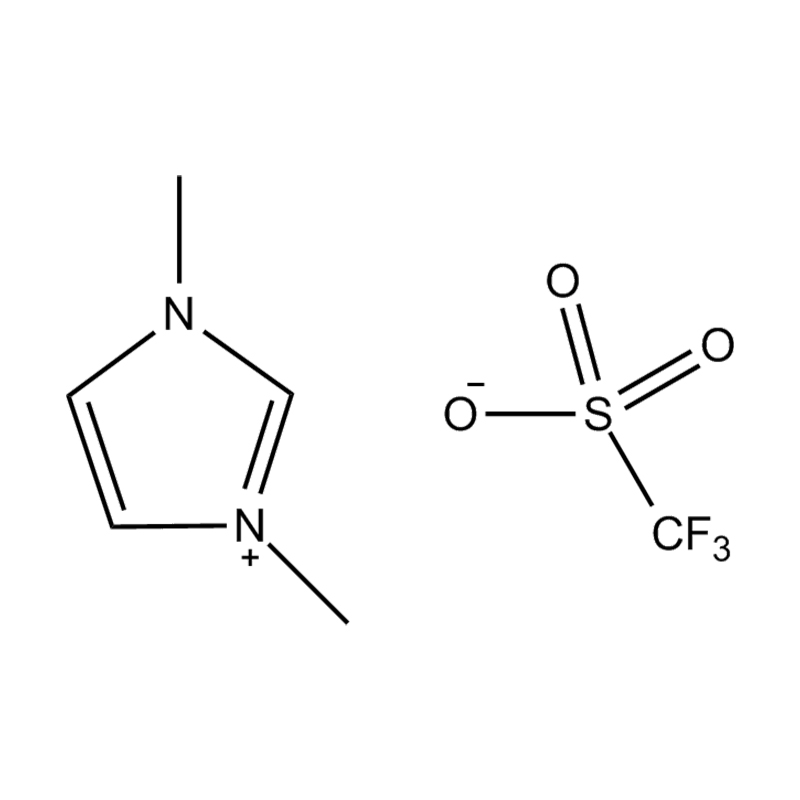
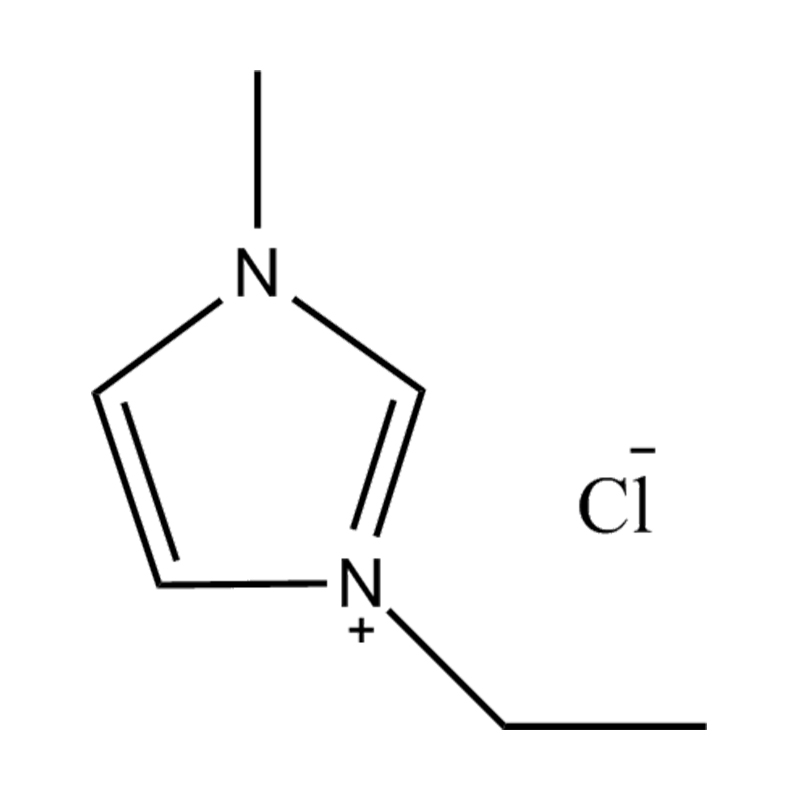
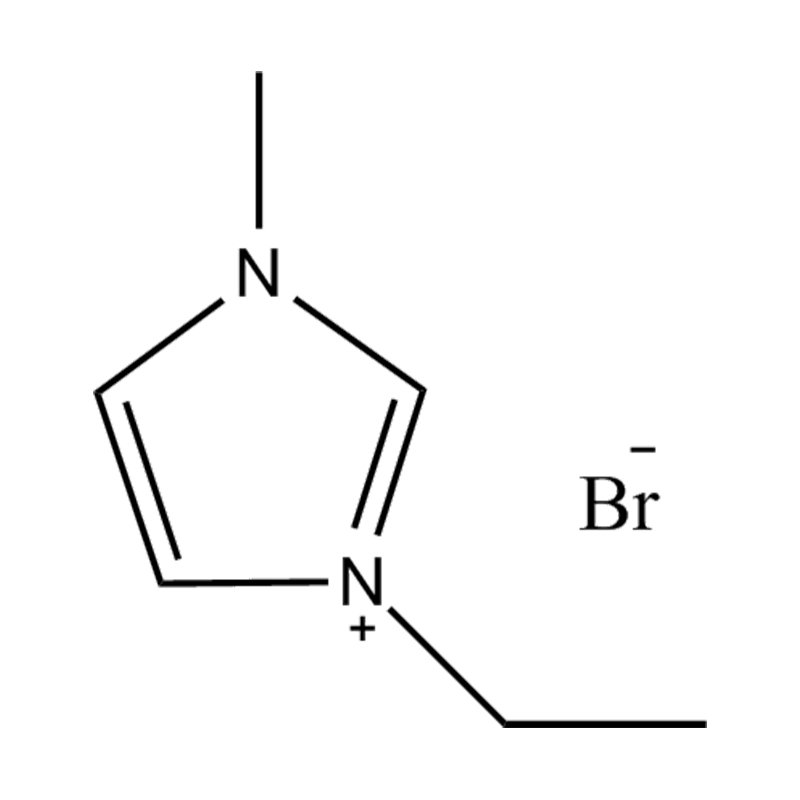
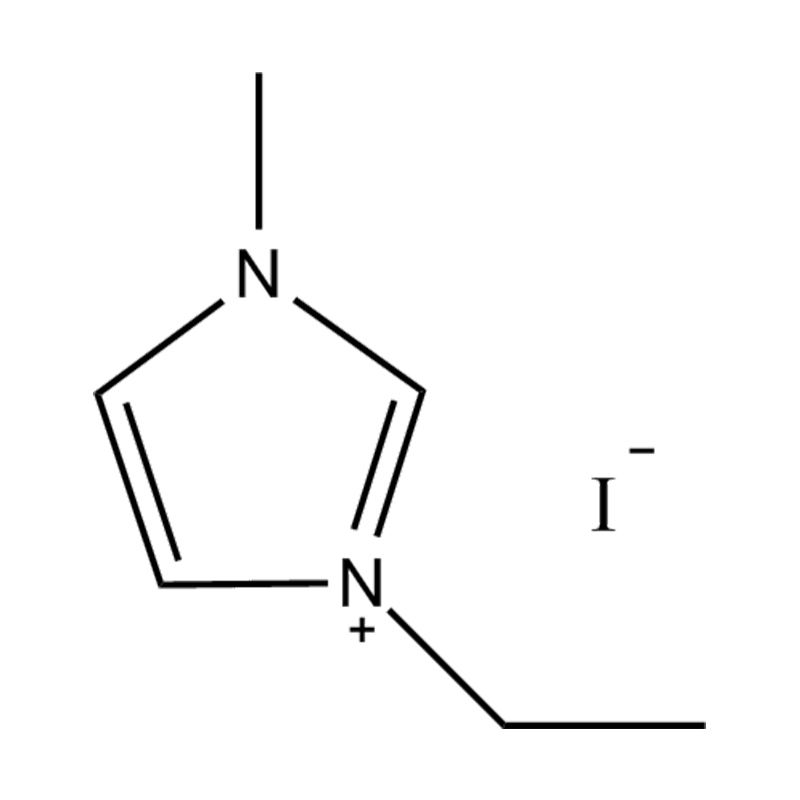
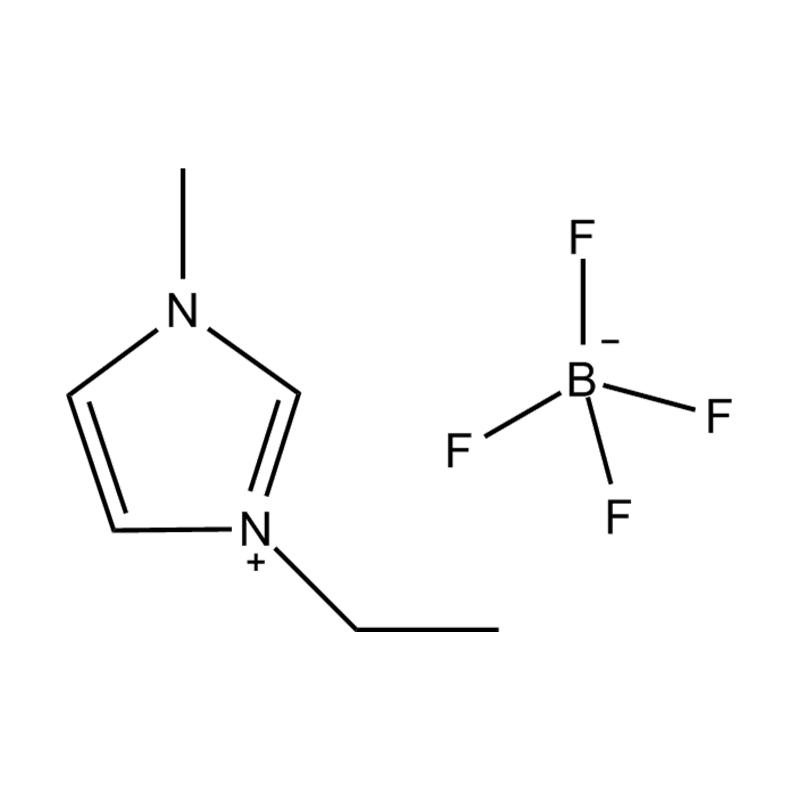
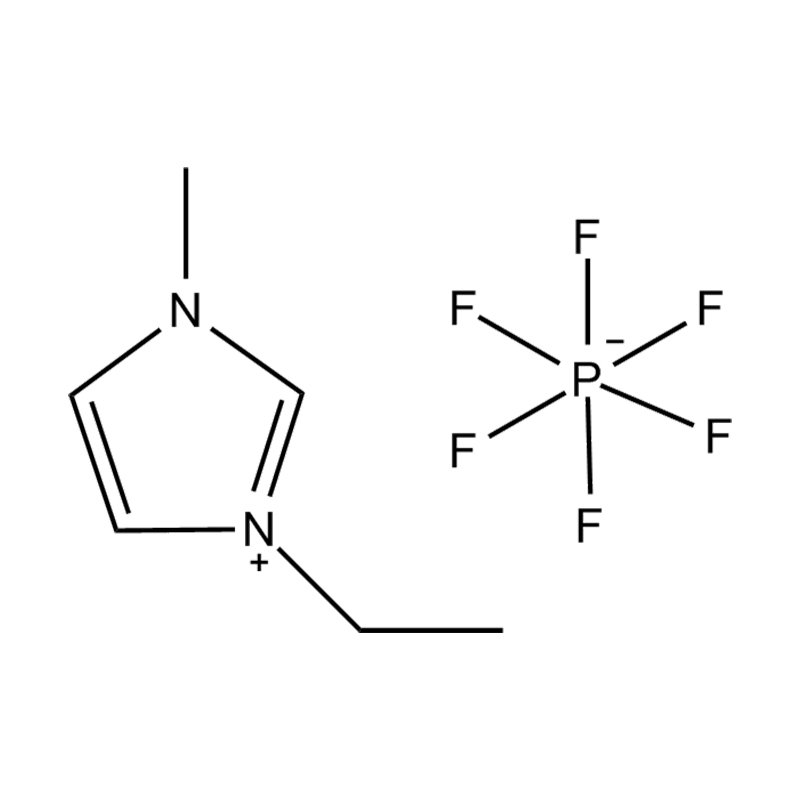
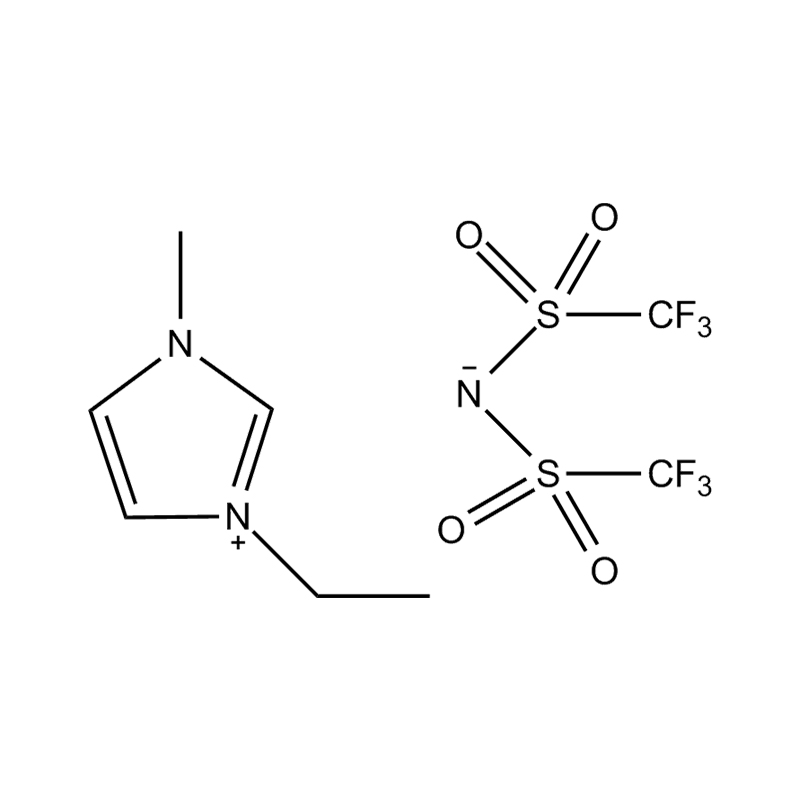
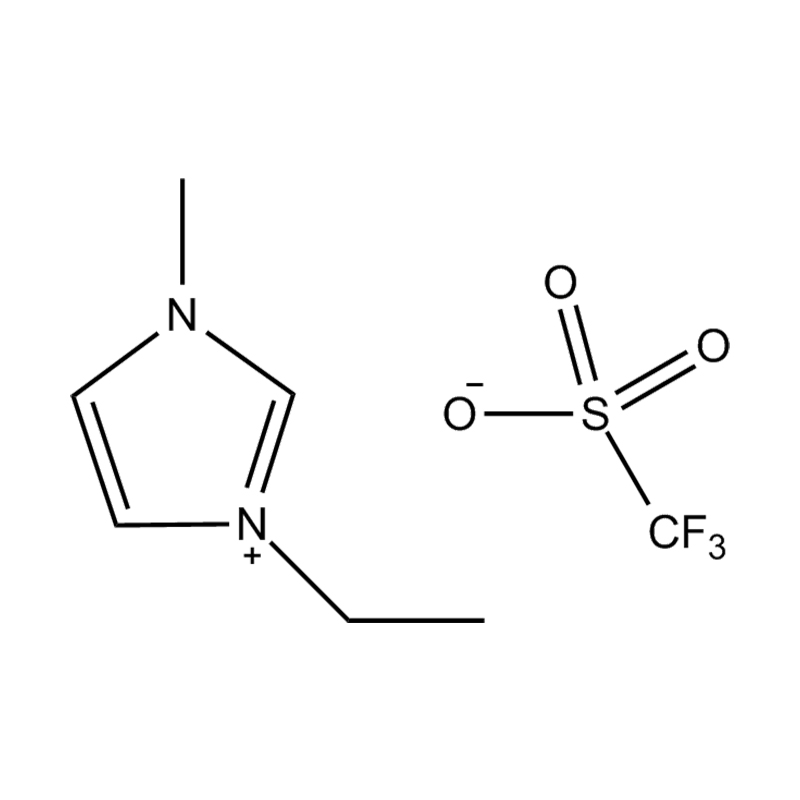
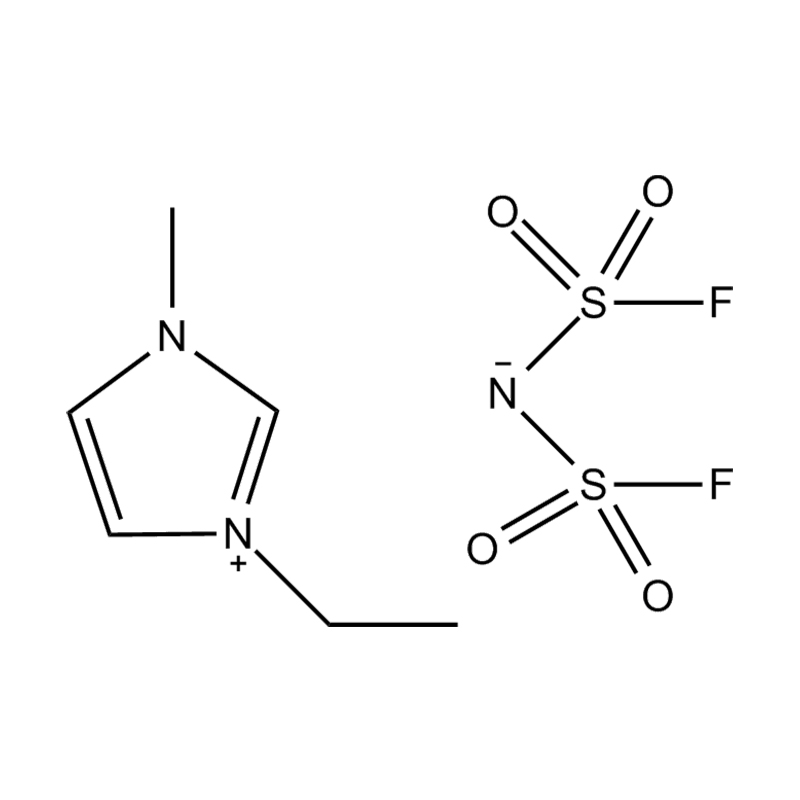
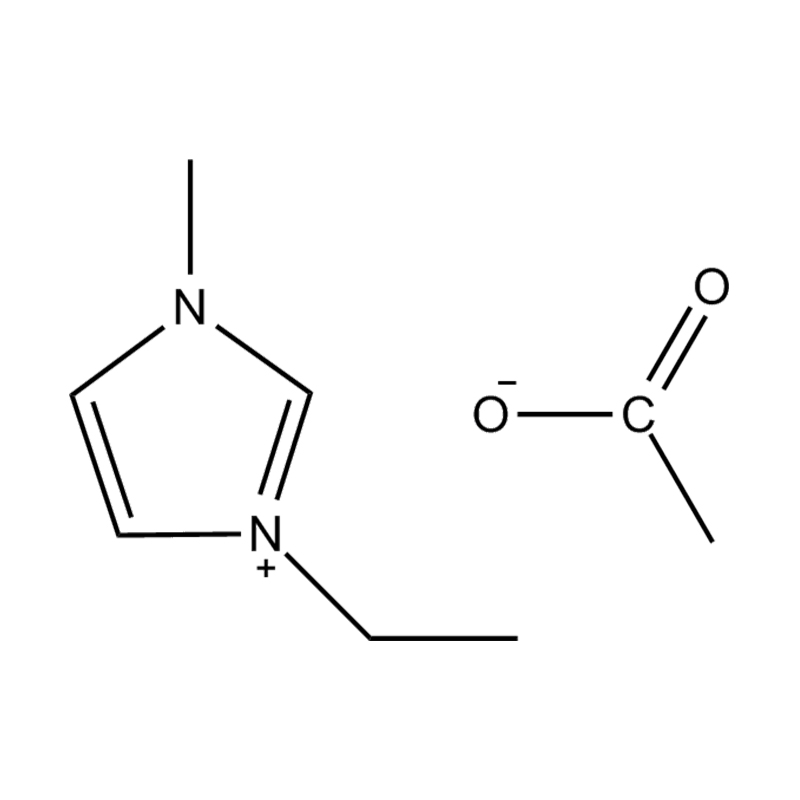
At the most fundamental level, an ionic liquid is a salt composed entirely of ions that remains in the liquid state below 100°C, often even at room temperature. Unlike traditional salts such as sodium chloride, which require high temperatures to melt, ionic liquids are typically made from bulky, asymmetrical organic cations (such as imidazolium, pyridinium, ammonium) paired with inorganic or organic anions (like bis(trifluoromethylsulfonyl)imide, PF₆⁻, BF₄⁻, or halides). The irregular shapes and weak coordination between ions prevent crystallization and result in their characteristic low melting points.
The physicochemical properties of ionic liquids are as diverse as their tunable molecular structures. One of their most defining traits is negligible vapor pressure, which makes them non-volatile and thus attractive as environmentally benign alternatives to traditional organic solvents. This feature alone has placed them at the forefront of green chemistry initiatives, where the elimination of volatile organic compounds (VOCs) is a priority.
Beyond being non-volatile, ionic liquids exhibit exceptional thermal and electrochemical stability. Many ILs can operate at temperatures exceeding 200°C without decomposing, and their wide electrochemical windows (up to 6V in some systems) make them ideal electrolytes in applications such as lithium-ion batteries, supercapacitors, and metal plating. Their intrinsic ionic nature also imparts high ionic conductivity, particularly in systems where conventional solvents would evaporate or degrade under harsh conditions.
Another critical advantage of ionic liquids lies in their chemical tunability. By modifying the cation or anion, scientists can fine-tune properties such as viscosity, polarity, hydrophilicity, or even coordination ability. This has enabled the creation of task-specific ionic liquids (TSILs) designed for highly selective roles—for instance, in CO₂ capture, biomass processing, or transition-metal catalysis. The modularity of ILs makes them a sort of "designer solvent" for complex chemical environments.
In the field of separations and extractions, ionic liquids offer several advantages over traditional solvents. Their ability to solubilize a broad range of organic and inorganic compounds, coupled with their immiscibility with water or hydrocarbons (depending on composition), enables highly efficient liquid-liquid extraction systems. ILs have been used for rare earth element recovery, removal of sulfur compounds from fuels, and even extraction of bioactive molecules from plants.
In catalysis, both as solvents and co-catalysts, ILs enhance reaction selectivity and yield while simplifying product separation. Many transition metal complexes exhibit improved stability and activity in IL media. Notably, ionic liquids have been utilized in asymmetric hydrogenation, alkylation, and cross-coupling reactions, often under milder conditions than in conventional systems.
One of the most cutting-edge applications of ionic liquids is in the realm of electrochemical devices and energy storage. IL-based electrolytes are being incorporated into lithium metal batteries, sodium-ion batteries, dye-sensitized solar cells (DSSCs), and even solid-state electrolytes. Their electrochemical inertness, non-flammability, and thermal tolerance offer critical advantages for improving both the safety and performance of energy systems.
Despite their promise, ionic liquids are not without challenges. Many ILs are still expensive to synthesize at scale, and some suffer from high viscosity, which limits mass transfer rates. Additionally, while ILs are often promoted as “green solvents,” their biodegradability and toxicity vary widely depending on structure, and long-term environmental impact remains an area of active research. Addressing these concerns through more sustainable synthesis routes and comprehensive life-cycle analysis will be essential for broader adoption.
The future of ionic liquids is increasingly interdisciplinary. In materials science, ILs are being used as solvents and templates in the synthesis of nanomaterials, metal-organic frameworks (MOFs), and conductive polymers. In biotechnology, they enable enzyme stabilization, protein extraction, and even DNA manipulation under non-traditional conditions. Their potential role in carbon capture and utilization (CCU) technologies is also gaining momentum, especially given their affinity for CO₂ and high thermal resistance.


 English
English Deutsch
Deutsch Español
Español 中文简体
中文简体